Regulatory approval in under 8 weeks possible for psychedelic trials in the UK
With the right compound and target indication, international drug developers may be eligible for ethical approval within 7 days and full approval in less than two months from regulatory submission.
The first code of ethics, famously known as Nuremberg Code, was formulated following the experiments conducted by Nazi doctors and scientists during World War II. Since then, the set of guidelines to safeguard the rights and wellbeing of research participants have come a long way, becoming more comprehensive and efficient each decade.
The UK has been proactive in looking at ways to accelerate clinical research whilst maintaining clinical excellence. For instance, approval times for clinical trials have halved since 2018. However, such a reduction hasn’t been reflected in the timelines of psychedelic trial approvals on a global scale. The UK is offering great opportunities to accelerate drug development, but many psychedelic drug developers are failing to utilise what is available to them. In this piece, we look at some of the reasons why this might be the case and how we can successfully navigate the pathway.
Psychedelics is a sector where innovative treatments are trialled in a complex regulatory environment. Drug developers need partners who know these barriers inside out to overcome them in the most efficient manner. The ethics board review has commonly been cited as one of the bottlenecks to the rapid approval of clinical trials and the latest changes to the fast-track review address this.
Timings for Initial Review Process
A complete set of regulatory feedback can now be provided in less than 30 days.
The regulatory and ethics review process in the UK
Building on the reformations introduced in 2021, the UK’s Health Research Authority (HRA) has made further changes to the way applications are handled during the fast-track review of clinical trials. This is in addition to the combined review, which allows research teams to submit a single application for both regulatory and ethics review at the same time leading to a single decision.
These latest changes accelerate the ethics review process by integrating their single fast-track Research Ethics Committee into a wider structure. This means that a larger pool of ethics committees, which currently includes 17 of them, will include fast-track applications as part of their usual meetings allowing ethics reviews to take place within one week of the trial package submission.
As a result of these changes, a complete set of regulatory feedback can be provided in less than a month from regulatory submission and a final outcome can be issued within 2 months.
The fast-track ethics review has been in effect since the 1st of August 2022. We’re already supporting our clients in this process here at Clerkenwell Health so that they can receive a complete set of regulatory feedback in less than a month of their regulatory submission.
Who is eligible?
The good news is that trials do not need to be led from the UK to be eligible. Fast-track ethics review is open to global clinical trials and phase I or I/II trials in healthy volunteers or patients. One specification is that trials need to be studying unapproved compounds (i.e. Clinical Trial of an Investigational Medicinal Product, CTIMP).
Clare Knight, Clerkenwell Health’s Senior Clinical Trials Manager believes that the UK’s regulatory environment is competitive on the global scale and that “the fast-track ethics approval is one of the many entrepreneurial avenues opened up by the regulators here, who have made it very clear that international drug developers should take advantage of this route too. For example, any CTIMP that is led from the UK but has another country participating, or any CTIMP that is taking place in another country and could be additionally placed in the UK is eligible for this scheme.”
With the introduction of the fast-track ethics review in addition to the Innovative Licencing and Access Pathway (ILAP) and combined review, psychedelic drug developers can significantly cut their drug development timelines in the UK. We work with drug developers to help them navigate these processes as part of the regulatory and scientific advisory services we offer.
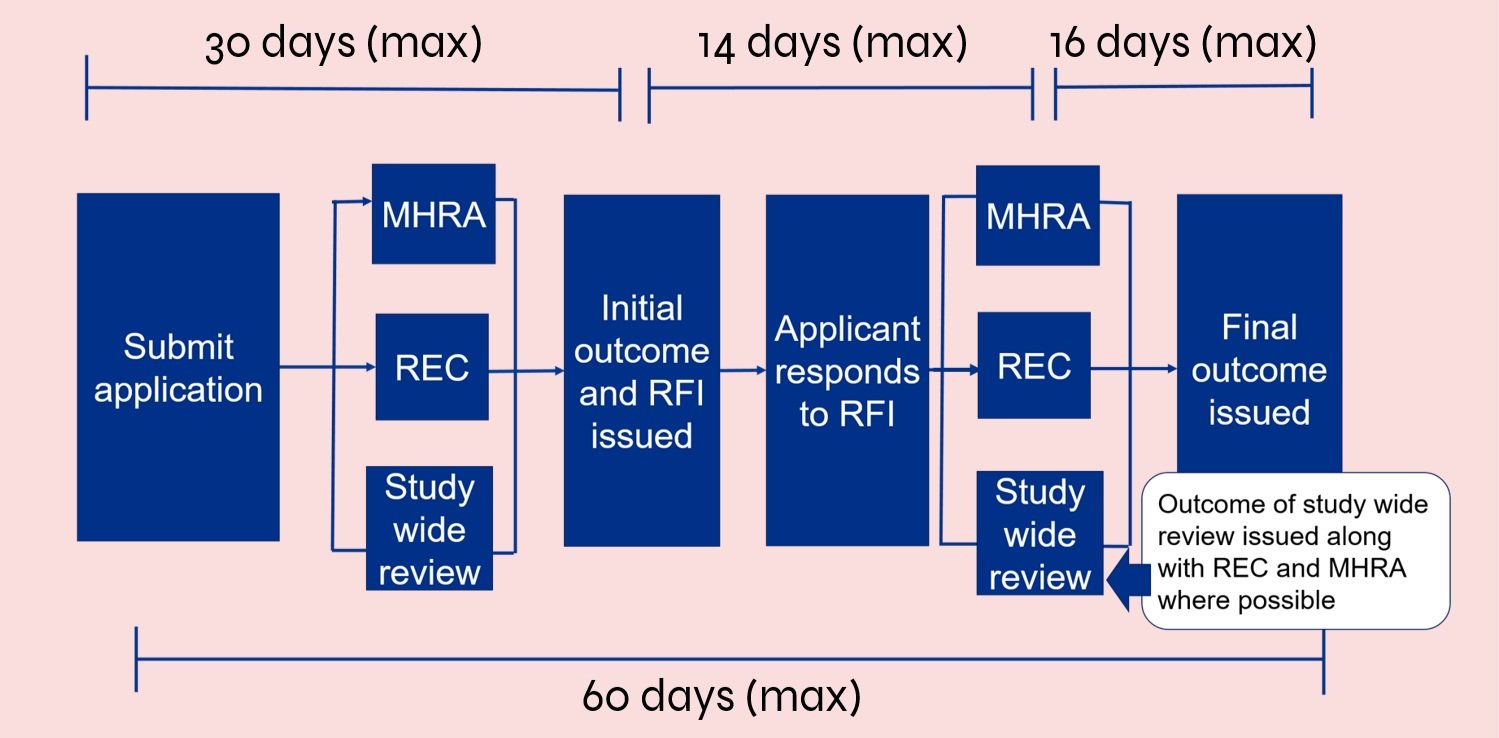
Review Process and Timelines
Maximum time from submission to outcome from Research Ethics Committee (REC) and Medicines and Healthcare products Regulatory Agency (MHRA) is 60 days: Research teams receive the initial response or request for further information (RFI) within 30 days from submission. This is followed by a 14-day period in which research teams respond to the feedback. MHRA and REC provide a final response within 16 days.
Source: NHS, Health Research Authority
Finding the right partner
Flexibility is one of the most important elements of a research relationship. Ability to pivot quickly or react to new challenges is a rarer virtue the larger an organisation. The value of Clerkenwell’s services stems from our adaptability to the needs of drug developers. We build capabilities for each drug developer we work with and deliver a tailored service.
Independence is key to operating at speed and having proper oversight of every step of a clinical trial. Operating our own clinical research site allows us to be more flexible in the way we design and deliver trials. Drug developers can influence the design of the facility and the way our therapists are trained.
Knight maintains that the “regulatory pathways are just one complexity facing drug developers looking to bring psychedelic compounds to market and as a CRO our job is to reduce those complexities”.
Through close collaboration with our clients and partners, we’re open to process innovation and trying novel approaches to pressing issues such as patient recruitment and therapist training. Our expert team work closely with drug developers to design cost-effective trials and ensure their trials are more efficient, ethical and informative.